
Global assembling of Academicians, Researchers, Scholars & Industry to disseminate and exchange information at 100+ Allied Academies Conferences
Session 01. Pharmaceutical Sciences
The drug sciences are a gathering of interdisciplinary spaces of study worried about the plan, activity, conveyance, and attitude of medications. They apply information from science (inorganic, physical, biochemical, and insightful), science (life systems, physiology, natural chemistry, cell science, and sub-atomic science), the study of disease transmission, measurements, chemometrics, math, physical science, and synthetic designing.
The drug sciences are additionally partitioned into a few explicit fortes, with four primary branches:
• Pharmacology: The investigation of the biochemical and physiological impacts of medications on individuals.
• Pharmacodynamics: The investigation of the cell and sub-atomic connections of medications with their receptors. Just "How the medication deals with the body"
• Pharmacokinetics: The investigation of the components that control the grouping of medication at different destinations in the body. Essentially "How the body deals with the medication"
• Pharmaceutical toxicology: The investigation of the destructive or poisonous impacts of medications.
• Pharmacogenomics: The investigation of the legacy of trademark examples of communication among medications and organic entities.
• Pharmaceutical science: The investigation of medication configuration to enhance pharmacokinetics and pharmacodynamics, and amalgamation of new medication atoms (Medicinal Chemistry).
• Pharmaceutics: The examination and plan of medication detailing for ideal conveyance, soundness, pharmacokinetics, and patient acknowledgment.
• Pharmacognosy: The investigation of prescriptions got from normal sources
Session 2: Drug discovery
This research topic aims to publish data on recent advances in early Drug Discovery against emerging/neglected tropical diseases, including parasitic, bacterial, viral, and fungal infections Here, we gather insightful data on the creation of fresh experimental methods, the discovery of fresh therapeutic plans, and the mode of action of traditional or cutting-edge antibacterial substances.
Over the past 40 years, the complexity of drug development has multiplied, necessitating the preclinical stage of drug research, an investigational new drug (IND) application, and extensive clinical testing before FDA marketing clearance. Successful Drug Discovery is like finding a safe and effective oasis in a chemical and biological desert. Because deserts are huge and oases are tiny, analyses based on the drug development strategy reveal that the discoverability of viable candidates is extremely sensitive to the accuracy of predictions. Especially since Drug Discovery is very difficult to measure late in the process before the project succeeds or fails.
Discovery Development
Preclinical research
Drug development process
Market monitoring
Session 3: Drug Development
Once a lead molecule has been found through the process of drug discovery, the process of drug development is used to bring a new pharmaceutical medicine to market. Preclinical research on microbes and animals is part of this process, as is requesting regulatory status, such as through the US Food and Drug Administration, to begin human clinical trials for an investigational new drug. It may also include the step of securing regulatory approval with a new drug application to market the drug. It normally takes more than ten years for a vaccine or medication to be approved, from concept to preclinical testing in the lab to clinical trial development, including Phase I-III trials.
Session 4: FDA Drug Review
The structural similarities between the FDA's drug reviews and many other regulatory bodies' judgments include high uncertainty, low reversibility, avoiding observable error, and high political stakes that encourage lobbying by interested parties. This essay investigates the policy implications of considering FDA drug evaluation as a politically motivated information processing activity. I contend that the incentives regulators face place restrictions on how quickly medication reviews may be completed, that these motivations may make privatization efforts difficult, and that political pressures may be helpful in identifying high-priority pharmaceuticals.
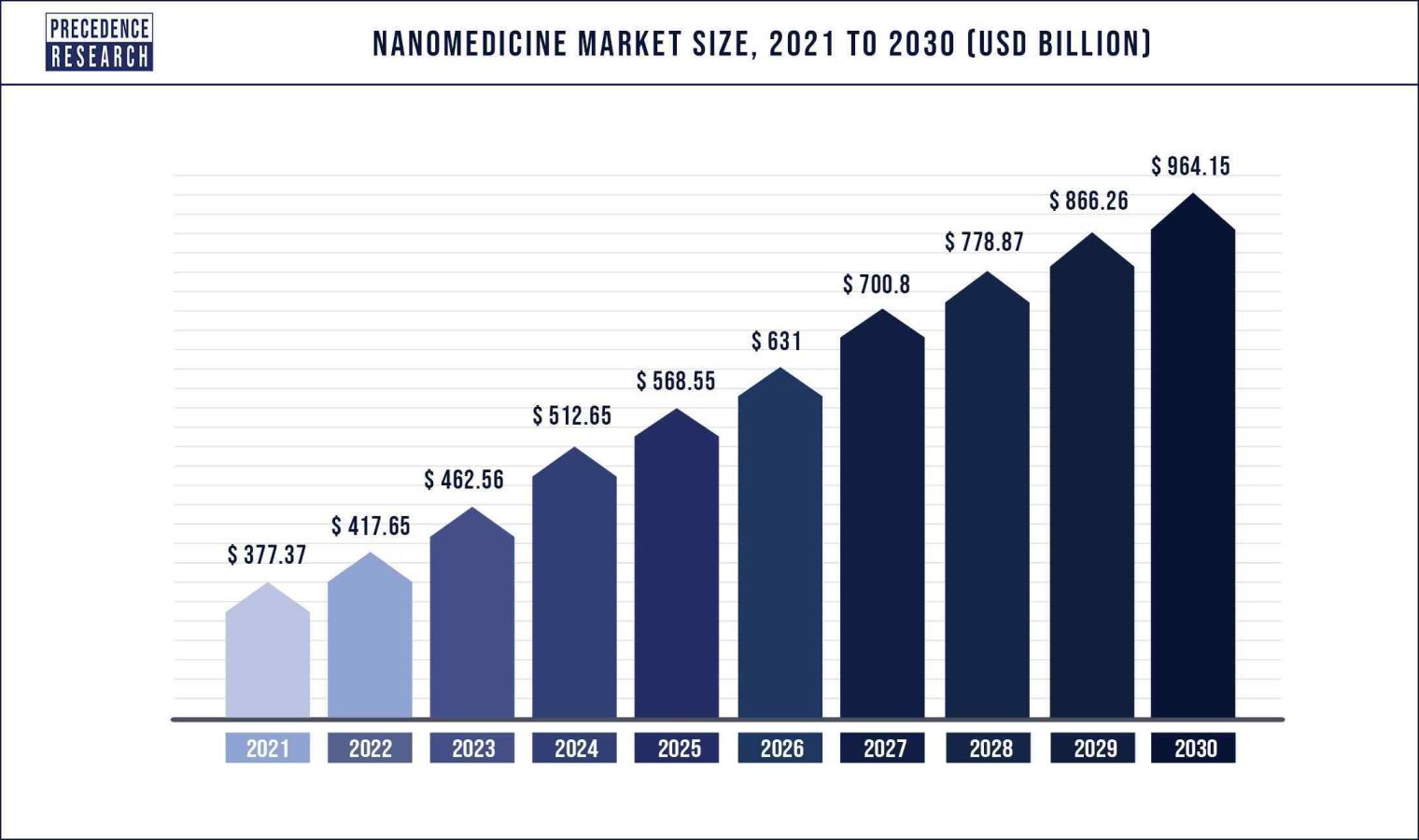
Growth Factor :
The global drug discovery market is significantly driven by the rising prevalence of various chronic diseases, rising healthcare expenditure, and patent expiration of certain popular drugs across the globe. The global population is facing growing burden of various diseases such as cardiovascular diseases, diabetes, cancer, respiratory diseases, and neurological disorders, which is significantly fueling the demand for the new and innovative drugs. The exponentially growing pharmaceutical industry owing to the rapid growth of the biopharmaceuticals is expected to drive the drug discovery market in the forthcoming years. The biopharmaceutical industry is growing at a rapid pace owing to the rising investments by the government and corporate for the development of new medicines to treat chronic diseases. Moreover, the favorable government regulations coupled with the active role of the authorities such as FDA and EMA is fueling the market growth.
The changing lifestyle, shifting consumption pattern, unhealthy food habits, rising pollution levels, and physical inactivity is resulting in the growing burden of various diseases like cancer, diabetes, and cardiovascular diseases. According to the International Agency for Research on Cancer, around 19.3 million new cancer cases and around 10 million cancer deaths were reported in 2020. As per the International Diabetes Federation, around 783 million people across the globe are estimated to live with diabetes by 2045. Further, over 1.2 million children and teenagers are suffering from type 1 diabetes. According to the World Health Organization, the cardiovascular diseases results in almost 18 million deaths each year and it is the leading cause of death across the globe. Thus, the demand for the innovative drugs that can cure chronic diseases is high among the global population, which in turn fuels the growth of the drug discovery market. The rising investments by the pharmaceutical companies in the research & development of various new drugs and clinical trials research is expected to propel the growth of the global drug discovery market.
Some of the prominent players in the global drug discovery market include:
Drug development 2021 was a fantastic success thanks in large part to the outstanding keynote speakers, attendees, students, media partners, and delegates who make the event an unforgettable one.
2021 December 8–9 saw the "4th International Conference on Drug Discovery and Drug Development". There were panel discussions with researchers and other academics during this two-day event as well as general keynote talks from research experts.
For the purpose of discussing drug discovery and its role in the healthcare, treatment, and prevention of the pandemic, Drug development 2021 provided a forum for all scientists, professional educators, cardiologists, surgeons, biomedical advisers, researchers, and clinical benefits specialists.
Eminent keynote speakers from numerous organizations and institutes shared their perspectives and social commentary throughout the conference, which had the topic "Pioneering the inventive heights in the field of drug discovery and development."
We warmly welcome you all from all over the world to attend the conference entitled “The International Conference on drug development scheduled during Jul 04-05, 2022, which is going to be held in Amsterdam, Netherlands.
Drug Development Conference 2023 will serve as a global forum for debating, exchanging, and exploring new fields of research and development as well as for learning about emerging technologies and algorithms in Drug development.
Drug Development Conference 2023 would be a fantastic chance to learn more about pharmacy-related topics and gain practical experience. A group of worldwide leaders in the developing pharmacy practice sectors will be gathered at this International Conference.
The program includes keynote addresses, lectures, interactive workshops, poster presentations, and exhibitions.
Keep in touch with us throughout Drug Development Conference 2023 to help us stay proactive and help Clinical Pharmacy's future take form.
We look forward to your presence in Amsterdam, Netherlands.
Best regards,
Drug Development Conference 2022,
Organizing Committee.
